precipitation method of gravimetric analysis|gravimetric stoichiometry formula step by : factories Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products). Resultado da SCREW translate: 金属物体, 螺丝(钉), 扭转,旋转, 监狱看守, 监狱看守(尤为犯人用语), 发生性关系, 性行为;性伙伴, 器具, 金属物体, 用螺丝固定(或拧紧), (用类似螺丝的东西)固定,拧紧.. Learn more in the Cambridge English-Chinese simplified Dictionary.
{plog:ftitle_list}
WEBNikolina Konstantinova Dobreva (born January 9, 1989), better known as Nina Dobrev, is a Canadian actress. Her first acting role was of Mia Jones in the drama series Degrassi: The Next Generation. She later became known for portraying Elena Gilbert and Katherine Pierce on The CW's supernatural drama series The Vampire Diaries. Born in Sofia and raised .
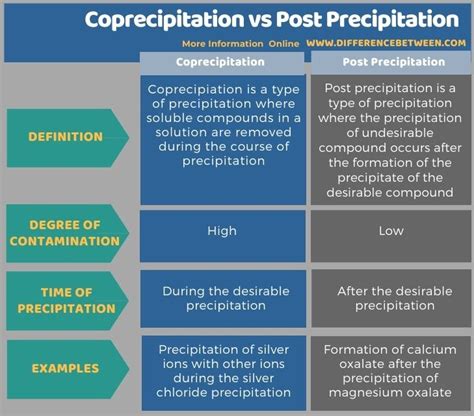
types of coprecipitation
All precipitation gravimetric analysis share two important attributes. First, the precipitate must be of low solubility, of high purity, and of known composition if its mass is to accurately reflect the .
Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. . Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products).
kf水分計
steps involved in gravimetric analysis
Some authors distinguish two sorts of gravimetry by precipitation: direct gravimetry, in which the analyte itself is weighed. For example, when nickel is determined by the weighing .
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate . Precipitation gravimetry is an analytical technique that uses the formation and mass of a precipitate to determine the mass of an analyte. It is important for the precipitate to be a .Precipitation gravimetry: The analyte is separated from a. solution of the sample as a precipitate and is converted to a compound of known composition that can be weighed. Volatilization .The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 C 2 O 4, is added to a measured, .
Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate . In gravimetric analysis, the substance to be determined (the analyte) is transformed quantitatively into an insoluble precipitate that is isolated and weighed.. Most of the time, the substance to be analyzed is transformed quantitatively into another that yields the precipitate that will be weighed. Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) . When the signal is the mass of a precipitate, we call the method precipitation gravimetry.
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. Gravimetric analysis is the process of isolating and measuring the weight of a particular element or compound. The major part of the gravimetric determination of compounds involves the . Gravimetric Analysis. Gravimetric analysis is a quantitative method in analytical chemistry wherein the concentration of a substance present in a sample is evaluated based on the measurement of its mass. This is done by precipitating the analyte (substance being analyzed) in a sample as a solid compound which is then separated, washed, dried and weighed.
wagner 910
Gravimetric analysis is a quantitative method in chemistry that involves determining the amount, or concentration, of a substance present in a sample based on the measurement of its mass. This .
4. Types of Gravimetric Analyses: • There are two main types of gravimetric analyses: A) Precipitation – analyte must first be converted to a solid (precipitate) by precipitation with an appropriate reagent. The precipitates from solution is filtered, washed, purified (if necessary) and weighed. B) Volatilization – In this method the analyte or its .
Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass.
Also common are gravimetric techniques in which the analyte is subjected to a precipitation reaction of the sort described earlier in this chapter. The precipitate is typically isolated from the reaction mixture by filtration, carefully dried, and then weighed (Figure \(\PageIndex{2}\)). . Combustion analysis is a gravimetric method used to .Gravimetric analysis is a method of a quantitative assessment of laboratory techniques based mostly on the dimension of an analyte's mass. . techniques are regularly convoluted and a minor misstep in a procedure can often mean tragedy for the analysis (colloid formation in precipitation gravimetry, for example). Measurement of mass is the . Precipitation gravimetry continues to be listed as a standard method for the determination of \(\text{SO}_4^{2-}\) in water and wastewater analysis [Method 4500-SO42– C and Method 4500-SO42– D as published in Standard Methods for the Examination of Waters and Wastewaters, 20th Ed., American Public Health Association: Wash- ington, D. C., 1998].established analytical methods we consider this term. Precipitation Gravimetry Gravimetric analysis is a standard classical method for determining the amount of a given component present in a host of solid and solution sample types. The method used here involves precipitating the component of interest from the unknown by means of an added reagent.
What Is Gravimetric Analysis GRAVIMETRIC ANALYSIS In precipitation gravimetry, the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, converted to a product of known composition by suitable heat treatment, and weighed. For example, a precipitation method forBefore we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, . When the signal is the mass of a precipitate, we call the method precipitation gravimetry. The indirect determination of PO 3 3 .
27. If a precipitate of known stoichiometry does not form, a gravimetric analysis is still feasible if we can establish experimentally the mole ratio between the analyte and the precipitate. Consider, for example, the precipitation .Questions on Sulfate Analysis. Approximately how many mL of 5% BaCl 2 2H 2 O solution would be required to precipitate all the sulfate if we assume that your samples are pure sodium sulfate? Assume that the density of the barium chloride solution is 1.00 g/mL. If the samples were pure potassium sulfate would you require a smaller or larger volume of barium chloride solution . Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 7.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction.
Gravimetric analysis is a method in analytical chemistry that uses mass measurement to quantify the quantity of an analyte (the ion being studied). The masses of two substances containing the analyte are compared in gravimetric studies. . Precipitation Method. In Precipitation Gravimetry, a known quantity of the substance that is to be .The four main types of this method of analysis are precipitation, volitilization, electro-analytical and miscellaneous physical method. . several organic functional groups or heteroatoms can be determined using gravimetric precipitation methods • Examples are outlined in Table 8.5 Example 1 • An ore containing magnetite, Fe3O4, was .Gravimetric analysis describes a set of methods in analytical chemistry for the quantitative determination of an analyte based on the mass of a solid.. In most cases, the analyte in solution is first converted to a solid by precipitation with an appropriate reagent. The precipitate can then be collected by filtration, washed to remove impurities, dried to remove traces of moisture from the . Example 1: Explaining Why Ashless Filter Paper Is Used in Gravimetric Analysis. In the precipitation method, why do we use ashless filter paper in chemical analysis? . As the name suggests, the precipitation method involves reacting an aqueous solution of our analyte with another aqueous solution to produce a precipitate. The precipitate that .
Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) . When the signal is the mass of a precipitate, we call the method precipitation gravimetry. - Optimize the precipitation conditions in order to obtain a desirable precipitate. - Learn how to do accurate measurents. . - Learn how to do calculations related to gravimetric analysis .
Features of Gravimetric Analysis •A given analyte is isolated from the sample and weighed in some pure form. •One of the most accurate and precise methods of macro quantitative analysis. •One of the oldest methods known (before 1810). •Absolute analysis (no standard needed). Weighing sample dissolving (heating-stirring)
Steps of a gravimetric analysis: precipitation, digestion, filtration, washing, drying, weighing, calculation The solubility product, the common ion effect Gravimetric calculations (key equations) . analysis Precipitation method Volatilization
precipitation method chemistry

Resultado da 4 de jan. de 2023 · How to give feedback, with examples. Giving constructive feedback can be tougher than you may assume and take some skills and .
precipitation method of gravimetric analysis|gravimetric stoichiometry formula step by